>>> The governments of several countries (plus the European Union and the United Nations) have programs for medical practitioners, members of the public, and pharmaceutical companies to report "adverse events" regarding prescription and over-the-counter drugs (as well as medical devices, personal care products, and occasionally other things).
As defined by the FDA, "adverse events" include hospitalization, surgical intervention, permanent disability/damage, "substantial risk of dying," death, birth defects, seizures, and more. These reports can also cover "product use errors, product quality problems, and therapeutic failures."
Several countries have set up publicly accessible databases containing details from these reports. The full reports themselves usually aren't there, but in some databases you'll find details from each report, such as type of adverse event, age and gender of the patient, etc. Other databases present overall statistics but not details of individual reports. You can bet that the pharmaceutical industry is not happy that these exist.
The Memory Hole 2 is totally supported by my readers. Please donate
These databases are far from complete or perfect, as every country admits. Reporting is mostly voluntary (there are some exceptions), and only a fraction of adverse events get reported. And there's usually no conclusive proof that any given event was directly caused by the drug. But when multiple reports of similar adverse reactions for the same drug start appearing, that's when this data become most useful.
Below are links to the searchable databases. If you know of others, please get in touch.
US Food and Drug Administration (FDA)
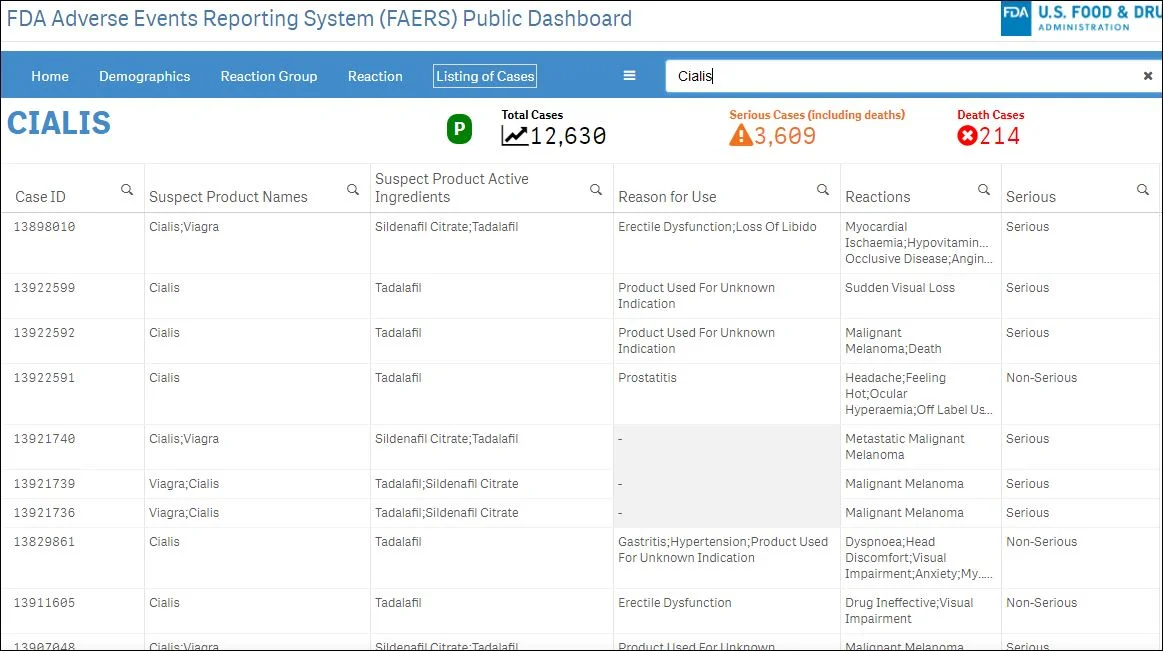
FAERS [Homepage/FAQ // Public Dashboard // API]: Adverse event reports for drugs (prescription and over-the counter). Currently contains over 14 million reports, including 1.4 million involving death and an additional 8 million classified as "serious."
VAERS [Overview // Data] Adverse event reports for vaccines
MAUDE: Adverse event reports for medical devices
CAERS: Adverse event reports for food, dietary supplements, and cosmetics (make-up, perfume/cologne, deodorant, tattoos, etc.)
ADE Reports: Adverse event reports for animal/veterinary drugs and devices
UN World Health Organization (WHO)
VigiAccess: "The data contains reports of suspected [adverse drug reactions] collected by national drug authorities in over 110 countries and span over more than 100 000 different medicinal products."
EU European Medicines Agency
EudraVigilance: Adverse events reports for drugs in every country in the European Union, plus Iceland, Liechtenstein, and Norway.
UK Medicines and Healthcare Products Regulatory Agency (MHRA)
YellowCard: Adverse event reports for drugs and medical devices
Health Canada
CVAROD: Adverse event reports for "health products" ("prescription and non-prescription medications; natural health products; biologics (includes biotechnology products, vaccines, fractionated blood products, human blood and blood components, as well as human cells, tissues and organs); radiopharmaceuticals; and disinfectants and sanitizers with disinfectant claims.")
Australia Therapeutic Goods Administration
DAEN: Adverse event reports for drugs and medical devices
New Zealand Medicines and Medical Devices Safety Authority
SMARS: Adverse event reports for drugs